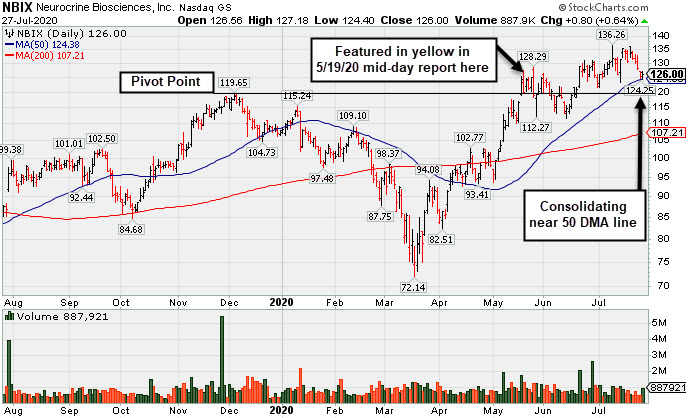
Consolidating Near 50-Day Moving Average; Earnings Report Due - Monday, July 27, 2020
Neurocrine Biosciences (NBIX +$0.80 or +0.64% to $126.00) found support today at its 50-day moving average ($124.38). Its color code was changed to green after rising back above its "max buy" level. Subsequent losses leading to a 50 DMA line violation would trigger a technical sell signal. Prior highs in the $119 area define the next important near-term support to watch on pullbacks.
Fundamentals remain strong. NBIX was last shown in this FSU section on 6/30/20 with an annotated graph under the headline, "Pulled Back Below "Max Buy" Level With Loss on Average Volume". It was highlighted in yellow with pivot point cited based on its 12/04/20 high plus 10 cents in the 5/19/20 mid-day report (read here). Progress has been limited and choppy since it technically broke out for a new 52-week high (N criteria) on the 5/18/20 with +101% above average volume behind a big gain after a gap up.
Fundamentals remain strong. NBIX was last shown in this FSU section on 6/30/20 with an annotated graph under the headline, "Pulled Back Below "Max Buy" Level With Loss on Average Volume". It was highlighted in yellow with pivot point cited based on its 12/04/20 high plus 10 cents in the 5/19/20 mid-day report (read here). Progress has been limited and choppy since it technically broke out for a new 52-week high (N criteria) on the 5/18/20 with +101% above average volume behind a big gain after a gap up.
Keep in mind that it is due to report Jun '20 quarterly results on 8/03/20. Volume and volatility often increase near earnings news. It reported earnings +545%, +200%, +163%, and +183% in the Jun, Sep, Dec '19 and Mar '20 quarters, well above the +25% minimum guideline (C criteria) versus the year ago periods, respectively. It showed greatly improved sales revenues up +89%, +46%, +86%, and +71% during that same span of quarterly comparisons. After years of losses it reported strong annual earnings (A criteria) results in FY '18 and '19.
There are 92.9 million shares outstanding (S criteria). The number of top-rated funds owning its shares rose from 651 in Jun '19 to 1,008 in Jun '20, a reassuring sign concerning the I criteria. Its current Up/Down Volume ratio of 1.3 is an unbiased indication its shares have been under slight accumulation over the past 50 days. It has an A Timeliness rating and a B Sponsorship Rating. It hails from the strong Medical - Biomed/Biotech industry which is currently rated 16th of the 197 industry groups listed in the newspaper (L criteria). In late April it received approval from the FDA for ONGENTYS, a new medication (N criteria) to help patients with Parkinson's disease better manage motor fluctuations.
Charts courtesy of www.stockcharts.com

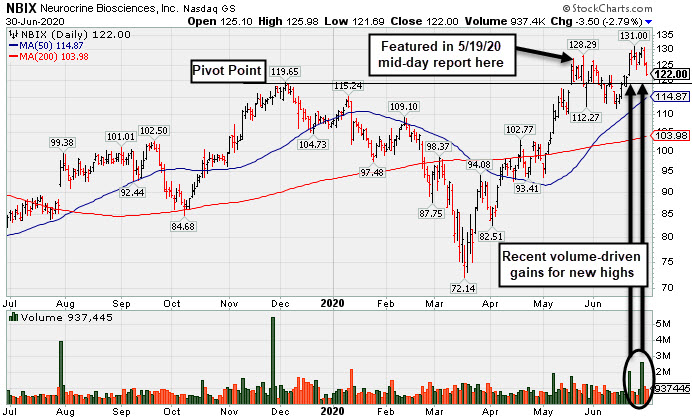
Pulled Back Below "Max Buy" Level With Loss on Average Volume - Tuesday, June 30, 2020
Neurocrine Biosciences (NBIX -$3.50 or -2.79% to $122.00) pulled back today with average volume, retreating from its all-time high. Its color code was changed to yellow after pulling back below its "max buy" level. Prior highs in the $119 area define important near-term support to watch above its 50-day moving average (DMA) line ($114.87). Fundamentals remain strong.
NBIX was last shown in this FSU section on 6/08/20 with an annotated graph under the headline, "Pullback Into Prior Base Negated Recent Breakout". It was highlighted in yellow with pivot point cited based on its 12/04/20 high plus 10 cents in the 5/19/20 mid-day report (read here). Technically, it broke out for a new 52-week high (N criteria) on the 5/18/20 with +101% above average volume behind a big gain after a gap up.
It reported earnings +545%, +200%, +163%, and +183% in the Jun, Sep, Dec '19 and Mar '20 quarters, well above the +25% minimum guideline (C criteria) versus the year ago periods, respectively. It showed greatly improved sales revenues up +89%, +46%, +86%, and +71% during that same span of quarterly comparisons. After years of losses it reported strong annual earnings (A criteria) results in FY '18 and '19.
There are 92.8 million shares outstanding (S criteria). The number of top-rated funds owning its shares rose from 651 in Jun '19 to 915 in Mar '20, a reassuring sign concerning the I criteria. Its current Up/Down Volume ratio of 1.7 is an unbiased indication its shares have been under accumulation over the past 50 days. It has an A Timeliness rating and a B Sponsorship Rating. It hails from the strong Medical - Biomed/Biotech industry which is currently rated 8th of the 197 industry groups listed in the newspaper (L criteria). In late April it received approval from the FDA for ONGENTYS, a new medication (N criteria) to help patients with Parkinson's disease better manage motor fluctuations.
Charts courtesy of www.stockcharts.com

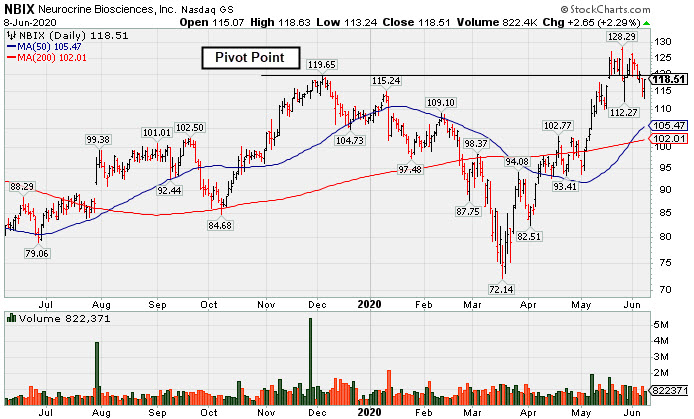
Pullback Into Prior Base Negated Recent Breakout - Monday, June 8, 2020
Neurocrine Biosciences (NBIX +$2.65 or +2.29% to $118.51) posted a gain today with below average volume. Its recent slump below the pivot point and back into the previously noted base raised concerns. Today's rebound was reassuring, but more damaging losses would not bode well.
NBIX was last shown in this FSU section on 5/19/20 with an annotated graph under the headline, "Biotech Firm Has New Parkinson's Treatment Drug Approved by FDA". It was highlighted in yellow with pivot point cited based on its 12/04/20 high plus 10 cents in the day's earlier mid-day report (read here). It reversed into the red and closed lower after early gains touched a new all-time highs (above the $126.98 in Sep '18). Technically, it broke out for a new 52-week high (N criteria) on the 5/18/20 with +101% above average volume behind a big gain after a gap up and its finished strong.
It reported earnings +545%, +200%, +163%, and +183% in the Jun, Sep, Dec '19 and Mar '20 quarters, well above the +25% minimum guideline (C criteria) versus the year ago periods, respectively. It showed greatly improved sales revenues up +89%, +46%, +86%, and +71% during that same span of quarterly comparisons. After years of losses it reported strong annual earnings (A criteria) results in FY '18 and '19.
There are 92.8 million shares outstanding (S criteria). The number of top-rated funds owning its shares rose from 651 in Jun '19 to 908 in Mar '20, a reassuring sign concerning the I criteria. Its current Up/Down Volume ratio of 1.4 is an unbiased indication its shares have been under accumulation over the past 50 days. It has an A Timeliness rating and a B Sponsorship Rating. It hails from the strong Medical - Biomed/Biotech industry which is currently rated 6th of the 197 industry groups listed in the newspaper (L criteria). In late April it received approval from the FDA for ONGENTYS, a new medication (N criteria) to help patients with Parkinson's disease better manage motor fluctuations.
Charts courtesy of www.stockcharts.com

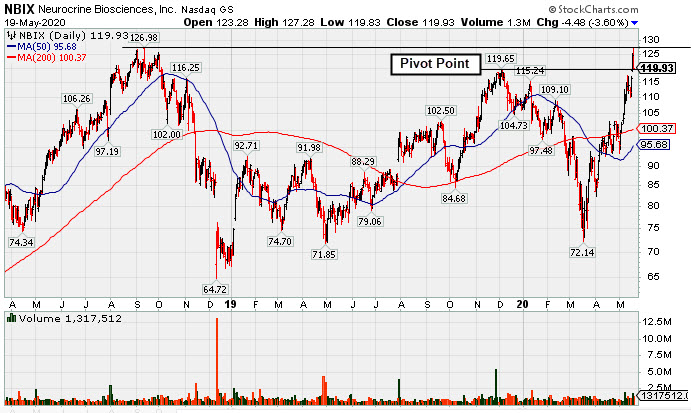
Biotech Firm Has New Parkinson's Treatment Drug Approved by FDA - Tuesday, May 19, 2020
Neurocrine Biosciences (NBIX -$4.48 or -3.60% to $119.93) was highlighted in yellow with pivot point cited based on its 12/04/20 high plus 10 cents in the earlier mid-day report (read here). It reversed into the red and closed lower after early gains touched a new all-time highs (above the $126.98 in Sep '18). Technically, it broke out for a new 52-week high (N criteria) on the prior session with +101% above average volume behind a big gain after a gap up and its finished strong. Subsequent gains into new all-time high territory could signal the beginning of a new substantial leg higher.
It reported earnings +545%, +200%, +163%, and +183% in the Jun, Sep, Dec '19 and Mar '20 quarters, well above the +25% minimum guideline (C criteria) versus the year ago periods, respectively. It showed greatly improved sales revenues up +89%, +46%, +86%, and +71% during that same span of quarterly comparisons. After years of losses it reported strong annual earnings (A criteria) results in FY '18 and '19.
There are 92.8 million shares outstanding (S criteria). The number of top-rated funds owning its shares rose from 651 in Jun '19 to 899 in Mar '20, a reassuring sign concerning the I criteria. Its current Up/Down Volume ratio of 1.6 is an unbiased indication its shares have been under accumulation over the past 50 days. It has an A Timeliness rating and a C Sponsorship Rating. It hails from the strong Medical - Biomed/Biotech industry which is currently rated 5th of the 197 industry groups listed in the newspaper (L criteria). In late April it received approval from the FDA for ONGENTYS, a new medication (N criteria) to help patients with Parkinson's disease better manage motor fluctuations..
Charts courtesy of www.stockcharts.com